
Arizona 3, California 5, Idaho 1, Illinois 2, Kentucky 1, Massachusetts 2, Maine 1, Michigan 1, Minnesota 2, North Carolina 2, New Jersey 1, Oregon 4, Pennsylvania 1, Rhode Island 1, Texas 8, Virginia 1, Washington 2, Wisconsin 1.
The FDA and CDC, in collaboration with the California Department of Public Health (CDPH), Infant Botulism Treatment and Prevention Program (IBTPP), and other state and local partners, continue to investigate a multistate outbreak of infant botulism. Epidemiologic and laboratory data show that ByHeart Whole Nutrition infant formula might be contaminated with Clostridium botulinum, which is causing infant illness in multiple regions of the country.
As of December 3, 2025, a total of 39 infants with suspected or confirmed infant botulism and confirmed exposure to ByHeart Whole Nutrition infant formula (various lots) have been reported from 18 states. Laboratory confirmation for some cases is ongoing. Illnesses started on dates ranging from August 9 to November 19, 2025. All 39 infants were hospitalized. No deaths have been reported to date. For 38 infants with age and 39 with sex information available, they range in age from 16 to 264 days and 15 (38%) are female.
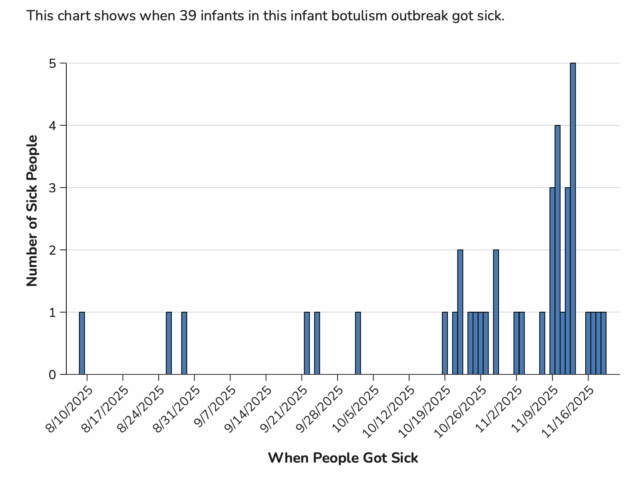
State and local public health officials are interviewing caregivers about the foods the infants were fed in the month before they got sick. Thirty-nine infants have been identified that were fed ByHeart Whole Nutrition powdered infant formula before getting sick.
FDA continues to receive reports that recalled formula is still being found on store shelves at Walmart, Target, Kroger, Acme, and Shaw’s, despite the ongoing recall of all ByHeart infant formula products. FDA continues to work with state partners and retailers to ensure an effective recall and immediate removal of these products from store shelves across the country. All ByHeart infant formula products have been recalled, and these products should not be available for sale in stores or online. This includes all formula cans and single-serve “anywhere pack” sticks.
Additional testing by ByHeart, FDA, CDC, and state partners is underway, and results are expected in the coming weeks. On November 19, ByHeart reported that they tested 36 samples of infant formula across three lots and five of those samples tested positive for Clostridium botulinum Type A. The specific lots that were tested have not been publicly reported by ByHeart. Positive sample results for finished product testing will be included and updated in the Sample Information section below.
FDA’s investigation is ongoing to determine the point of contamination. This advisory will be updated as information becomes available.
Case Count Map Provided by CDC

Case Counts
Total Illnesses: 39 (2 New)
Hospitalizations: 39 (2 New)
Deaths: 0
Last Illness Onset: November 19, 2025
States with Cases: AZ, CA, ID, IL, KY, MA, ME, MI, MN, NC, NJ, OR, PA, RI, TX, VA, WA, WI
Product Distribution: Online and nationwide (including Guam and Puerto Rico), and internationally
Useful Links
- CDC Outbreak Advisory
- Infant Botulism Treatment and Prevention ProgramExternal Link Disclaimer
- ByHeart Expanded Recall Announcement
Product Images



Sample Results
Product sampling and testing is being conducted by FDA, CDC, state partners, and ByHeart. Available information on positive samples is included below. This table will be updated as additional results become available or are shared with FDA.
Due to the large number of samples, only positive results are being reported here. The detection of Clostridium botulinum in infant formula is complex, and a negative test result does not rule out the presence of the bacteria in the product.
Parents and caregivers should not use any ByHeart infant formula, regardless of test results.
| Sample Collected/Analyzed by | Product | Test Result | Toxin Type |
| CDPH | Opened container of ByHeart Infant Formula (Batch No. 251131P2) | Positive | Type A |
| ByHeart | ByHeart Infant Formula (Batch/Batches Not Reported) | Positive | Type A |
| ByHeart | ByHeart Infant Formula (Batch/Batches Not Reported) | Positive | Type A |
| ByHeart | ByHeart Infant Formula (Batch/Batches Not Reported) | Positive | Type A |
| ByHeart | ByHeart Infant Formula (Batch/Batches Not Reported) | Positive | Type A |
| ByHeart | ByHeart Infant Formula (Batch/Batches Not Reported) | Positive | Type A |
International Distribution
The ByHeart infant formula recall impacts markets outside the United States. Customer information provided by Amazon shows that a limited quantity of recalled ByHeart infant formula was distributed to Argentina, Brazil, Brunei, Canada, Chile, China, Colombia, Ecuador, Egypt, Hong Kong, Israel, Jamaica, Japan, Republic of Korea, Peru, Philippines, Romania, Singapore, South Africa, Thailand, and the British Virgin Islands.
Consumers worldwide should not use any ByHeart brand infant formula as all ByHeart products are included in this recall.