CDC, public health and regulatory officials in several states, and the U.S. Food and Drug Administration (FDA) investigated a multistate outbreak of Listeria monocytogenes infections.
Epidemiologic, laboratory, and traceback data showed that supplement shakes manufactured by Prairie Farms were contaminated with Listeria and made people sick.
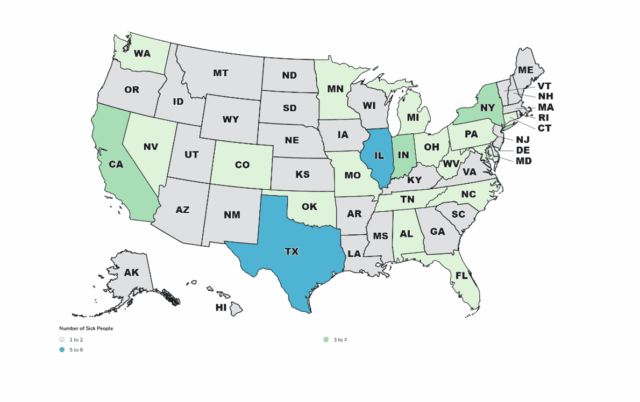
A total of 42 people infected with the outbreak strain of Listeria monocytogenes were reported from 21 states. Illnesses started on dates ranging from August 17, 2018, to March 13, 2025. Of 42 people with information available, 41 were hospitalized. A total of 14 deaths were reported from 9 states: California, Illinois, Indiana, Michigan, Minnesota, North Carolina, New York, Tennessee, Texas, and Washington. Most people in this outbreak reported living in long-term care facilities or were hospitalized prior to becoming sick.
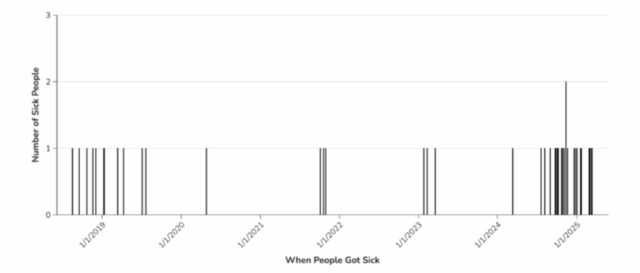
CDC investigated this outbreak in 2018, 2021, and 2023. Epidemiologic data in previous investigations identified that sick people were residents in long-term care facilities and nursing homes and the likely source was a food served in those types of institutions, but there was not enough information to identify a specific food. CDC reopened the investigation in October 2024 after six new illnesses were reported.
State and local public health officials interviewed people about the foods they ate in the month before they got sick. Of 42 people with information, 38 (90%) reported living in long-term care facilities or were hospitalized before becoming sick. Nine people reported mechanical soft diets, including foods like supplement shakes. Information provided by facilities showed at least 4 people drank the recalled supplement shakes. Additionally, facilities confirmed that supplement shakes were available to residents.
FDA traced food records from long-term care facilities. These records showed that supplement shakes made by Prairie Farms were a common food served at the long-term facilities. On February 4, 2025, FDA conducted an onsite inspection and collected environmental samples and product samples at Prairie Farms Dairy, Inc. in Fort Wayne, Indiana. Three of the environmental swabs collected from the processing area tested positive for Listeria monocytogenes. Whole genome sequencing (WGS) analysis determined that the Listeria detected in these samples was closely related to the strain of Listeria that caused illnesses in this outbreak. See Investigation Update: Listeria Outbreak, Supplement Shakes, February 2025 | Listeria Infection | CDC; See also Outbreak Investigation of Listeria monocytogenes: Frozen Supplemental Shakes (February 2025) | FDA; See also, FDA Executive Incident Summary, CARA #1281 dated 4/24/2025.
On February 22, 2025, Lyons Magnus LLC recalled 4 oz. Lyons ReadyCare and Sysco Imperial frozen supplement shakes with best by dates of 022125 to 022126 (February 21, 2025 – February 21, 2026). See recall notice, Lyons Magnus Recalls Lyons ReadyCare and Sysco Imperial Frozen Supplemental Shakes Manufactured by Third Party Because of Possible Health Risk | FDAThe causal link between John Wills’ infection with Listeria monocytogenes and supplemental shakes produced by Prairie Farms Dairy, Inc. is clear. John was a patient at Richmond Post Acute Care (RPAC) facility beginning on May 6, 2024, and remained there until July 21, 2024, when he was transferred to Kaiser Hospital because of symptoms consistent with meningitis and bacteremia.
A blood specimen collected on July 21, 2024, was positive for Listeria monocytogenes at Kaiser Regional Laboratory. The California Department of Health Microbial Diseases Laboratory (CDPH MDL) confirmed this result, CDPH MDL Specimen ID Number M24S000172. Whole Genome Sequencing (WGS) analysis determined the allele code (LM01.1-13.1.2.6.247) and assigned PulseNet ID number PNUSAL022839 to this result. Review of NCBI data showed that John Wills was a genetic match to patients infected with a strain of Listeria monocytogenes associated with consumption of Prairie Farms Dairy, Inc. supplemental shakes, CDC Outbreak 1812MLGX6-1.
Contra Costa County Health Department staff interviewed John Wills and learned he was a resident at Richmond Post Acute Care Facility during his incubation period for listeriosis. Health department records document that the food service provider to Richmond Post Acute Care facility was Sysco Food Services, a known vendor of Prairie Farms Dairy, Inc. supplemental shakes. Thus, it is more likely than not that John Wills consumed contaminated Prairie Farms Dairy, Inc. supplemental shakes while he was a patient at Richmond Post Acute Care Facility leading towards his infection with Listeria monocytogenes.